PLASMID DNA

BACKGROUND
The increased use of gene and cell therapy, e. g. for immunotherapies with modified T cells, leads to a higher demand for recombinant viral vectors. These types of therapies are based on the use of adeno-associated viruses (AAV), lentiviruses or other virus types. Plasmid DNA is used here as starting material for the transient expression.
Due to the clinical success and the growing number of late-phase clinical studies, the demand for plasmid DNA is increasing too. As a result, there is a need for larger manufacturing capacities. In order to meet the requirements of late-phase clinical trials and in-market demands for successful products, a significant change in manufacturing approaches for the cell therapy itself and viral vector production is necessary. New technical solutions are needed to cope with these changes.
To meet the demand for niche therapies (less than 10.000 patients) the installed small production platform, producing 10 g per batch, seems sufficient. For a marketed product a reasonable prediction is 100 – 1.000 g plasmid vector per year. Production platforms are therefore in need of significant scale up.
THE CHALLENGE
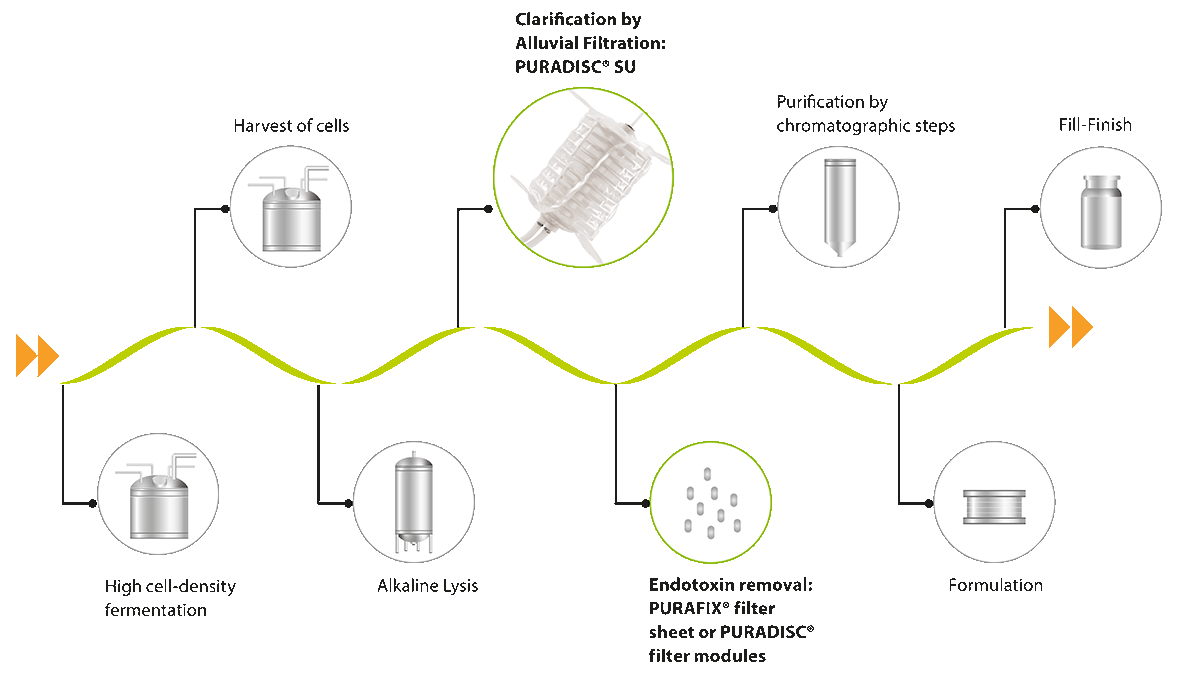
Usually, high cell density fermentation processes run in fed-batch mode. The generated cell paste is harvested, e. g. by centrifugation, and the cells then lysed by physico-mechanical (e. g. homogenization) or chemical (e. g. alkaline lysis) treatment. The removal of cell debris and impurities, such as non-plasmid DNA and endotoxins, is the biggest challenge in the first clarification step. An efficient and scalable technology can minimize the need of further purification steps. The technology of choice for this is alluvial filtration.
THE SOLUTION
The PURADISC® SU system is the state-of-the-art technology for alluvial filtration. It is based on depth filters that capture contaminants in their three-dimensional matrix. The used PURAFIX® depth filter sheets are low in extractable ions and pyrogens. By adding filter aid, such as diatomaceous earth, the capacity per m2 filter area can be drastically increased.
Product Range
| Product | Size | Description | Batch size | Best use |
| PURACAP® SU | 2″ Capsule | Lab scale | Up to 2 L | Process development, evaluation of filter sheet and filter aid grade |
| PURACAP® SU | 5″ Capsule | Small pilot scale | Up to 10 L | Evaluation of 2″ results, clarification process development |
| PURACAP® SU | 10″ Capsule | Pilot scale | Up to 25 L | Evaluation of lab scale results; small production scale |
| PURADISC® SU | 12M Module | Pilot scale | Up to 100 L | Evaluation of lab scale results; small production scale |
| PURADISC® SU | 12S Module | Bio process scale | Up to 200 L | Production scale |
| PURADISC® SU | 12D Module | Bio process scale | Up to 500 L | Production scale |
| PURADISC® SU | 16D Module | Bio process scale | Up to 1000 L | Production scale |
| PURAFIX® line | Filter sheet | Endotoxin reduction | From lab to production |
Reduction of endotoxins in pre-clarified solutions |
Save one Process Step
The main advantage of alluvial filtration for the clarification step is the elimination of the two-step approach. Due to the increased filtration capacity the traditional approaches such as centrifugation followed by depth filtration or a two-step depth filtration are not necessary anymore. The simultaneous reduction of impurities e. g. HCP is another advantage.
Flexible Optimization
Different grades of filter aid and PURAFIX® filter sheets can be combined and thus enable you to optimize the respective production process.
Linearly Scalable
The PURADISC® SU system is linearly scalable from process development up to production scale (PURADISC® SU 16D modules). The transfer from method development stage to large-scale processes can be easily achieved due to linear scalability.
Related Products